The FDA Approval of Molnupiravir Would Be a Gamechanger. But Isn't There Already a 'Covid Pill' on the Market?
The FDA panel approval of Molnupiravir is set to shake up the Covid therapeutics market. But is it just 'repackaged Ivermectin'?
A COVID-19 pill is poised to hit the U.S. market after a Food and Drug Administration panel narrowly recommended that Merck's drug Molnupiravir be granted Emergency Use Authorization.
The FDA panel voted 13-10 that the antiviral drug’s benefits outweigh its risks. If the FDA grants the EUA, Molnupiravir could become first drug that Americans could take at home to treat the coronavirus.
There are major implications if the FDA grants approval to Molnupiravir. First, it signals a shift towards therapeutics as becoming a more prominent tool in the ongoing fight against Covid.
Second, it raises questions about the necessity of Covid vaccine mandates, particularly as the federal vaccine mandates continues to incur legal setbacks. If the mRNA therapies marketed as 'vaccines' continue to fail to halt the transmission of variants such as delta and omicron, then it will further call into question their efficacy and reliability.
Third, Molnupiravir may prove to be a safe and effective alternative to vaccines in mild-to-moderate Covid cases. Since the risk profile for Covid varies greatly by age, and is impacted by factors such as obesity and immunocompromisation, the FDA authorization would provide an ample alternative for younger, healthy Americans to choose to forego the Covid prophylactic shots.
Fourth, there are many people who believe that Molnupiravir and Ivermectin, both manufactured by Merck, are essentially the same product. This will be unpacked in some detail below.
It is important to at least give a cursory look at the FDA briefing document that guided the panel discussion on Tuesday.
"This Advisory Committee briefing document summarizes the data submitted to support the Emergency Use Authorization (EUA) of molnupiravir (MOV) for the treatment of mild-to-moderate coronavirus disease 2019 (COVID-19) in adults who are at high risk for progression to severe COVID-19, including hospitalization or death," the FDA briefing document states.
"Currently three antivirals monoclonal antibodies (mAbs) are authorized for the treatment of mild-to-moderate COVID-19 in individuals who are at high risk for progression to severe COVID-19, including hospitalization or death. These products all require intravenous (IV) or subcutaneous (SC) injection for administration. MOV is an oral prodrug with antiviral activity against severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2)," the FDA briefing document added.
The FDA briefing document can be read in full below:
The entire FDA panel meeting can be viewed in full below. It is encouraged that people take at least a minute or two to view the decision-making process:
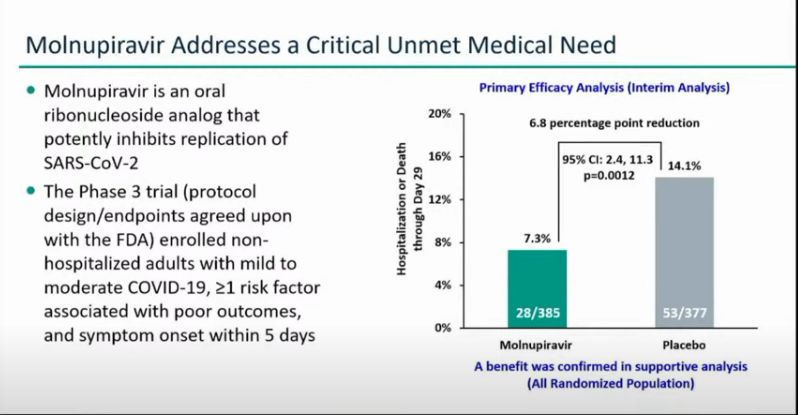
At 59:00 into the FDA meeting, Dr. Sean Curtis, the Senior Vice President of Global Regulatory Affairs and Clinical Safety for Merck, said the clinical data on Molnupiravir showed impressive efficacy and safety.
"Molnupiravir was shown to significantly reduce risk of hospitalization or death by approximately 50%," Dr. Curtis said. Furthermore, the clinical data showed that "through day 29, no deaths were shown in patients who received Molnupiravir, as compared to 8 deaths in patients who received placebo."
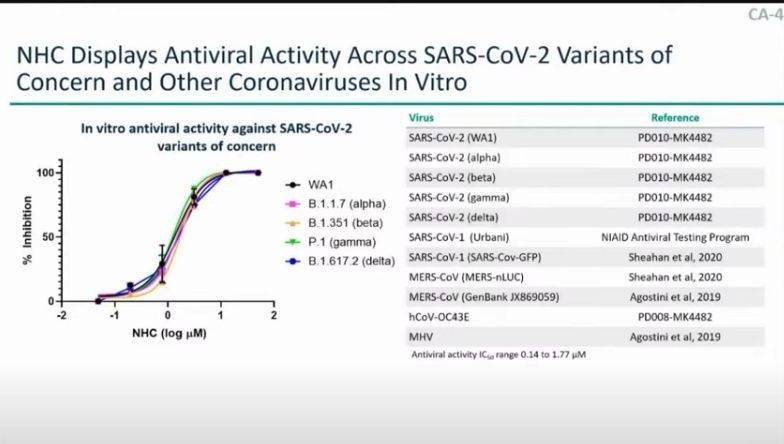
At approximately 1:02:00 into the meeting, Dr. Daria Hazuda, PhD., who leads research for infectious diseases and vaccines at Merck, explained the mechanism of action for Molnupiravir. Dr. Hazuda gets into the technical chemistry of Molnupiravir, before explaining that the oral treatment has similar efficacy across a broad spectrum of SARS-CoV-2 variants. (Note: Clinical results for the Omicron variant were not discussed.)
"Note that the antiviral activity is similar across SARS-CoV-2 variants of concern, including alpha, beta, gamma, delta, as well as mu," Dr. Hazuda said.
This is great, but isn’t there already a “Covid pill” on the market?
There is a fascinating point being raised about whether Molnupiravir is just 're-packaged' Ivermectin. This will be discussed in some detail below.