The CDC Announces Investigation into Pfizer Covid Vaccine Over Elevated Risk of Stroke in Elderly Patients
There is also new evidence that Pfizer knew there was a potential link from the start.
Breaking update: The CDC and FDA have concluded an “extensive” 24-hour investigation and have concluded that there is “nothing to see here.”
The Centers for Disease Control and Prevention, nearly two years after the launch of the Covid "vaccines," on Friday said that it has finally decided to investigate whether or not they cause an elevated risk of strokes.
The CDC said on Friday a preliminary Covid vaccine “safety signal” had been identified and it is now investigating whether the mRNA shots create an increased risk of ischemic stroke in people 65 and older.
In its statement on Friday, the CDC said the preliminary signal hasn’t been identified with Moderna's vaccine.
“Following the availability and use of the updated (bivalent) COVID-19 vaccines, CDC’s Vaccine Safety Datalink (VSD), a near real-time surveillance system, met the statistical criteria to prompt additional investigation into whether there was a safety concern for ischemic stroke in people ages 65 and older who received the Pfizer-BioNTech COVID-19 Vaccine, Bivalent,” the CDC said.
“Rapid-response investigation of the signal in the VSD raised a question of whether people 65 and older who have received the Pfizer-BioNTech COVID-19 Vaccine, Bivalent were more likely to have an ischemic stroke in the 21 days following vaccination compared with days 22-44 following vaccination.”
According to the CDC, an ischemic stroke “occurs when blood clots or other particles block the blood vessels to the brain.”
In its statement, the CDC claimed that a large study of updated bivalent vaccines from Pfizer-BioNTech “using the Centers for Medicare and Medicaid Services database revealed no increased risk of ischemic stroke.”
In a statement to Fox News Digital, a spokesperson for Pfizer said, “Pfizer and BioNTech have been made aware of limited reports of ischemic stroke that have been observed in the CDC Vaccine Safety DataLink (VSD) database in people 65 and older following vaccination with the Omicron BA.4/BA.5-adapted bivalent COVID-19 Vaccine by Pfizer and BioNTech.”
The CDC isn’t recommending a change in vaccine practice; however, it had earlier acknowledged that thrombosis with thrombocytopenia syndrome (TTS) is a "rare but serious adverse event" associated with the Covid vaccines. The syndrome causes blood clots or issues with clotting, which present the danger of causing a stroke.
A June 2022 study in the Journal of Stroke and Cerebrovascular Diseases, the official journal of the National Stroke Association, did a review of the "increasing reports of various types of stroke including ischemic stroke, and hemorrhagic stroke, as well as cerebral venous sinus thrombosis (CVST) after COVID-19 vaccination."
It counseled physicians to "be aware of the possible stroke after COVID-19 vaccination to ensure rapid diagnosis and treatment," the study said. "CVST [cerebral venous sinus thrombosis] is an important phenomenon that may occur after COVID-19 vaccination and is mostly associated with VITT [Vaccine-induced immune thrombotic thrombocytopenia]. The diagnosis of VITT-associated stroke should be made with high suspicion because of its rapid and diverse clinical manifestations."
"Stroke should be considered when a patient develops any neurological complaints, especially constant headaches, within 4 weeks of COVID-19 vaccination," the study added.
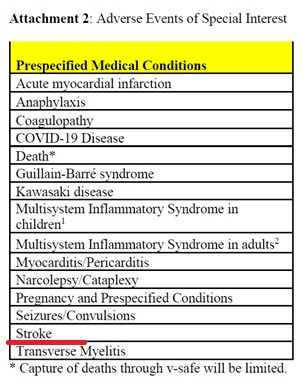
Furthermore, newly obtained documents in Freedom of Information Act litigation by the Informed Consent Action Network show that the CDC's "prespecified medical conditions" chart — including heart attacks, myocarditis, stroke and spinal-cord inflammation — appeared in the first version of the v-safe protocol Nov. 19, 2021, before the vaccines launch, ICAN's lawyer Aaron Siri wrote.
Here is a list of Adverse Events of Special Interest that Pfizer was tracking in v-safe prior to the vaccine and surveillance system launch.
Pfizer removed “stroke” and the rest of these conditions from the check-the-box list, Siri reported, thus suggesting that it was trying to cover up these associated AESIs.
The dam is breaking. The truth is finally catching up to the lies.



Why limit the study to 65 and older? Sounds like they are already getting ready to write the conclusion: "Well, it's hard to say. They're old and they're not very healthy to begin with, so there are too many factors involved to conclude anything."
One of several things is going to happen here.
1. They will whitewash this and claim it was only in this specific high risk group.
2. They will scream indignantly that the toxicity data was withheld from them as the FDA advisory group last week.
3. They will find someone to throw under the bus and blame all if it on them.